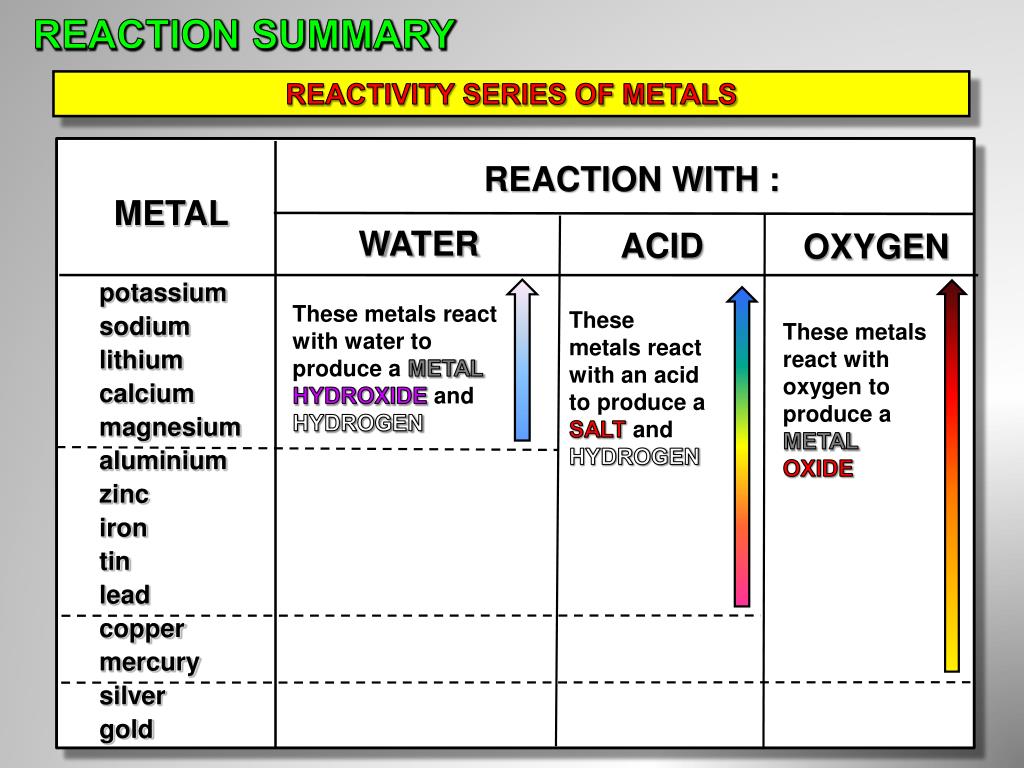
Which Metal Is More Reactive Mercury Or Copper . Alkali metal are most reactive metals. Francium is most reactive element in periodic table. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. At the bottom are the least reactive metals. 2 na (s) + 2. 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: More reactive metals displace less reactive metals from their compounds and. Because of their low ionization energies, they easily. The metal that does the replacing is always more reactive than the replaced metal. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 9 rows the reactivity series ranks metals by how readily they react.
from www.slideserve.com
Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. Francium is most reactive element in periodic table. More reactive metals displace less reactive metals from their compounds and. 2 na (s) + 2. Alkali metal are most reactive metals. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. The metal that does the replacing is always more reactive than the replaced metal. Because of their low ionization energies, they easily.
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563
Which Metal Is More Reactive Mercury Or Copper At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. At the bottom are the least reactive metals. Alkali metal are most reactive metals. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. More reactive metals displace less reactive metals from their compounds and. 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: Because of their low ionization energies, they easily. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. 9 rows the reactivity series ranks metals by how readily they react. The metal that does the replacing is always more reactive than the replaced metal. Francium is most reactive element in periodic table. 2 na (s) + 2.
From www.quelpr.com
CSEC Chemistry Reactivity of Metals Which Metal Is More Reactive Mercury Or Copper Francium is most reactive element in periodic table. More reactive metals displace less reactive metals from their compounds and. Alkali metal are most reactive metals. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 9 rows the reactivity series ranks metals by how readily they react. Because of their low ionization energies, they easily. At the. Which Metal Is More Reactive Mercury Or Copper.
From brainly.in
what is meant by reactivity series of metal? Give an examples Brainly.in Which Metal Is More Reactive Mercury Or Copper Francium is most reactive element in periodic table. 9 rows the reactivity series ranks metals by how readily they react. Because of their low ionization energies, they easily. 2 na (s) + 2. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. Alkali metals such. Which Metal Is More Reactive Mercury Or Copper.
From frosdasset.weebly.com
Most reactive metal on periodic table frosdasset Which Metal Is More Reactive Mercury Or Copper The metal that does the replacing is always more reactive than the replaced metal. Because of their low ionization energies, they easily. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. More. Which Metal Is More Reactive Mercury Or Copper.
From poot1961.blogspot.com
Most Reactive Metal In Periodic Table Poot1961 Which Metal Is More Reactive Mercury Or Copper At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. 9 rows the reactivity series ranks metals by how readily they react. At the bottom are the least reactive metals. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons. Which Metal Is More Reactive Mercury Or Copper.
From www.periodictableprintable.com
What Is The Most Reactive Element In The Periodic Table 2024 Periodic Which Metal Is More Reactive Mercury Or Copper At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. The metal that does the replacing is always more reactive than the replaced metal. 2 na (s) + 2. At the bottom are the least reactive metals. Because of their low ionization energies, they easily. More reactive metals displace. Which Metal Is More Reactive Mercury Or Copper.
From www.adda247.com
Reactivity series of Metals and Non Metals Which Metal Is More Reactive Mercury Or Copper At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. 2 na (s) + 2. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. At the bottom are the least reactive metals. Francium is most reactive element in periodic table. The metal that does the. Which Metal Is More Reactive Mercury Or Copper.
From www.teachoo.com
Reactivity Series of Metals Chart [and How to remember] Teachoo Which Metal Is More Reactive Mercury Or Copper At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. The metal that does the replacing is always more reactive than the replaced metal. Alkali metal are most reactive metals. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. Because of their low ionization energies,. Which Metal Is More Reactive Mercury Or Copper.
From utedzz.blogspot.com
Periodic Table Reactivity Series Of Metals Class 10 Periodic Table Which Metal Is More Reactive Mercury Or Copper The metal that does the replacing is always more reactive than the replaced metal. 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and. 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: Francium is. Which Metal Is More Reactive Mercury Or Copper.
From www.documentarytube.com
What are the most Reactive Metals? DocumentaryTube Which Metal Is More Reactive Mercury Or Copper 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and. 26 rows metals with a greater total number of electrons tend to be more reactive as. Which Metal Is More Reactive Mercury Or Copper.
From www.periodictableprintable.com
Most Reactive Metal On Periodic Table 2024 Periodic Table Printable Which Metal Is More Reactive Mercury Or Copper Francium is most reactive element in periodic table. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. More reactive metals displace less reactive metals from their compounds and. 9 rows the reactivity series ranks metals by how readily they react. Because of their low ionization. Which Metal Is More Reactive Mercury Or Copper.
From infoupdate.org
Periodic Table Activity Series Of Metals Which Metal Is More Reactive Mercury Or Copper 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 9 rows the reactivity series ranks metals by how readily they react. Francium is most reactive element in periodic table. At the top. Which Metal Is More Reactive Mercury Or Copper.
From www.geeksforgeeks.org
Reactivity Series Reactivity of Metals, Features, Tricks Which Metal Is More Reactive Mercury Or Copper 9 rows the reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and. 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: At the bottom are the least reactive metals. Alkali metal are most reactive metals. Because of. Which Metal Is More Reactive Mercury Or Copper.
From getrevising.co.uk
The Reactivity Series Revision Notes in GCSE Chemistry Which Metal Is More Reactive Mercury Or Copper 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. More reactive metals displace less reactive metals from their compounds and. 58 rows the most. Which Metal Is More Reactive Mercury Or Copper.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Which Metal Is More Reactive Mercury Or Copper 58 rows the most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: Alkali metal are most reactive metals. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 9 rows the reactivity series ranks metals by how readily they react. Because of their low ionization energies, they easily.. Which Metal Is More Reactive Mercury Or Copper.
From isseark.weebly.com
Most reactive metals on the periodic table isseark Which Metal Is More Reactive Mercury Or Copper Alkali metal are most reactive metals. More reactive metals displace less reactive metals from their compounds and. Francium is most reactive element in periodic table. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which. Which Metal Is More Reactive Mercury Or Copper.
From revisechemistry.uk
Reactivity of Metals AQA C4 revisechemistry.uk Which Metal Is More Reactive Mercury Or Copper 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. The metal that does the replacing is always more reactive than the replaced metal. At the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. 58. Which Metal Is More Reactive Mercury Or Copper.
From morganthorpe.z13.web.core.windows.net
Periodic Table Reactivity Chart Which Metal Is More Reactive Mercury Or Copper Alkali metal are most reactive metals. Alkali metals such as potassium, sodium, and calcium are all highly reactive elements. Francium is most reactive element in periodic table. 2 na (s) + 2. At the bottom are the least reactive metals. The metal that does the replacing is always more reactive than the replaced metal. 58 rows the most reactive metals,. Which Metal Is More Reactive Mercury Or Copper.
From www.compoundchem.com
The Metal Reactivity Series Compound Interest Which Metal Is More Reactive Mercury Or Copper 9 rows the reactivity series ranks metals by how readily they react. Francium is most reactive element in periodic table. 26 rows metals with a greater total number of electrons tend to be more reactive as their outermost electrons (the ones which will be. Because of their low ionization energies, they easily. 58 rows the most reactive metals, such as. Which Metal Is More Reactive Mercury Or Copper.